News
GLP-1 Users Lose Weight, and Their Taste for Meat
Research•3 min read
News
A growing body of evidence links antibiotic overuse in meat production to infections in humans.

Words by Natasha Gilbert
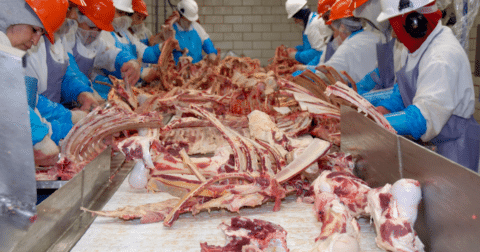
Factory farming is known to contribute to the global threat of antimicrobial-resistant bacteria. But scientists don’t fully understand which steps in factory farming lead to resistance or how likely these resistant bacteria from food animals are to reach people and make them sick.
Two new studies help to fill these gaps. One study from researchers at Dicle University in Turkey found that beef slaughterhouses are a reservoir of antibiotic-resistant extraintestinal pathogenic Escherichia coli (ExPEC) — a strain of E. coli bacteria that is the leading cause of urinary tract infections, or UTIs.
One quarter of all workers’ hands tested positive for ExPEC, and more than half of the bacterial samples were resistant to at least one antimicrobial drug, the researchers found.
In a separate study, food animals were the likely source of 18% of antimicrobial-resistant UTIs in patients sampled in Southern California.
Overuse of antimicrobial medicines in intensive farming is among the main drivers of antimicrobial resistance — a scourge that kills 1.27 million people a year, topping deaths from HIV and malaria combined, according to the World Health Organization.
Many more antimicrobials are sold for use in farm animals than for humans each year in high-income countries, research suggests. They are typically used to treat and prevent disease and sometimes to promote growth.
The more antimicrobial medicines are used, the more chances bacteria and other pathogenic organisms like fungi and viruses have to evolve resistance to the drugs. Veterinary scientists and animal welfare advocates worry that overusing antimicrobials in farm animals is used to compensate for poor husbandry.
“Raising billions of animals in filthy, crowded conditions can lead to diseases like ExPEC, and those fecal bacteria don’t just stay on the farms, they pollute the air, water, land, and ultimately end up on grocery store shelves,” Andrew deCoriolis, Executive Director of Farm Forward, an advocacy group pushing to end factory farming, tells Sentient in an email.
The veterinary medicine industry argues that antimicrobial resistance is low among animals and plays down the possibility that resistance can spread from animals to people.
In the new study published in the journal Veterinary Sciences in September, researchers collected 447 samples from two small slaughterhouses located in the southeast of Turkey. The samples included swabs from the beef carcasses, rectal samples, hides, workers’ hands and knives used to cut up the carcasses.
The researchers found that around 8% of the samples were contaminated with ExPEC. Globally, this E. coli strain is the leading cause of UTIs in people. This strikes Steve Roach, an analyst for the Food Animal Concerns Trust and Keep Antibiotics Working, advocacy groups fighting misuse of antibiotics in farm animals, as high.
The study also found that one quarter of all workers’ hands tested positive for the bacteria. This suggests that workers might not only infect themselves but also spread the bacteria within the slaughterhouse and into the wider community.
Slaughterhouses “play a critical role” in interactions between humans, animals and the environment, the researchers write in the study. This suggests “multiple potential cross-contamination points” that could help transmit the bacteria into the food chain, the researchers write.
In addition, over half of the ExPEC samples were found to be resistant to at least one antimicrobial drug from a selection of three or more different agents. These include ciprofloxacin and cephalosporins, which the WHO categorizes as the highest-priority medicines that are critically important to protecting human health.
Roach is concerned at the level of infection and resistance to antimicrobial medicine detected in the study.
“Eight percent of isolates containing virulence genes associated with human infections seems high. When you combine it with the high level of resistance … to drugs used to treat ExPEC, it creates a very real public health risk,” Roach tells Sentient in an email.
The study focuses on slaughterhouses in Turkey, where the system and regulation may differ from that in the United States. Nonetheless, the results are broadly informative. The basic science of bacterial infection and the development of antimicrobial resistance remain the same, writes Roach.
Other new research published in October found that food animals were an important source of UTIs in people tested in Southern California. The study included 23,483 bacteria samples from people with UTIs during a 4-year period ending in 2021. The researchers genetically sequenced just under one quarter of these.
The study found that 18% of the UTIs were caused by ExPEC that likely originated from food animals. The study authors suggest people could have become infected through eating or handling contaminated meat.
Other reports show that ExPEC is widely resistant to important antibiotics including cephalosporins and fluoroquinolones. The growing resistance has slowed treatment of infected patients, leading to more illness and deaths, write the researchers.
Previous studies have found this strain of E. coli in some farm animals including pigs, dairy cattle and chicken. But there is little global data on the strain in beef cattle. In one of the few studies available, researchers from the United States Department of Agriculture (USDA) in Nebraska found that just 0.37% of 542 E. coli samples from beef cattle hides were the ExPEC variety. The researchers concluded that beef was not a major reservoir of ExPEC. The study was funded in part by a beef industry group in Nebraska.
Still, a growing body of research implicates industrial animal farms as an important source of dangerous human infections, writes deCoriolis.
Either high levels of antimicrobial resistance or pathogenic strains of bacteria on their own would create health risks, writes Roach. “When you combine them, it is even worse,” he writes.
To reduce risks to human and animal health, regulators would need to reduce antibiotic use in food animals. The U.S. Food and Drug Administration should track the use of antibiotics on farms and set mandatory targets for reducing the use of the most medically important drugs, writes deCoriolis.
Under former President Joe Biden, pressure from the U.S. government weakened global commitments to tackle antimicrobial resistance (AMR), according to nonprofit newsroom U.S. Right to Know. The United States objected to targets to cut the quantity of antimicrobials used in agriculture by at least 30% from countries’ current levels by 2030. The targets were proposed in an early version of a United Nations political declaration on antimicrobial resistance. The targets were absent from the final draft of the political declaration, dated 9 September 2024. Instead, global leaders promised to “meaningfully reduce” the amount of antimicrobials used in animal agriculture.
“Recent administrations of both parties have utterly failed at holding the meat and pharmaceutical companies accountable for the incredible danger they are putting us all in,” writes deCoriolis.